The effects of an anti-inflammatory diet alone or in combination with acupuncture on mental health, anthropometric indices, and metabolic status in diabetic patients with depression: a randomized, controlled clinical trial
In this present randomized controlled clinical trial, we examined the effects of an anti-inflammatory diet, both alone and in combination with acupuncture, on mental health, anthropometric indices, and metabolic status in diabetic patients with depression.
Eligibility criteria
The inclusion criteria were as follows: (i) both genders, (ii) aged 20–75 years, (iii) diagnosed with T2DM for more than six months, (iv) currently taking oral anti-diabetic medications, and (v) experiencing mild-to-moderate depression. Patients were excluded if they were using insulin or liraglutide, taking medicinal herbs or herbal remedies, antibiotics, antioxidant supplements (including multivitamins and minerals, vitamin E, vitamin C, and zinc), suffering from severe depression, having cardiovascular diseases, chronic liver or kidney disease, untreated thyroid disorders, or any other diabetes-related complications that could lead to inflammatory and oxidative conditions. Additionally, individuals with other types of diabetes or those taking psychiatric medications were excluded. Pregnancy and lactation were also considered exclusion criteria.
Intervention
Eligible patients were randomly selected from those referred to a diabetes clinic affiliated with Tehran University of Medical Sciences. Before the trial commenced, the study was explained to the eligible individuals, and if they volunteered to participate, they signed a written informed consent form.
Eligible individuals were assigned to one of three groups: (i) an individualized anti-inflammatory diet combined with acupuncture therapy, (ii) an individualized anti-inflammatory diet alone, or (iii) a control group receiving standard treatment and dietary recommendations. Importantly, all three groups received standard treatment, which included medications, a weight-loss diet for overweight and obese participants, and general healthy dietary recommendations, throughout the duration of the study. Patients were instructed to report any changes in the type and dosage of their medications during the study period.
Diet
For each participant, an individualized diet tailored to their individual characteristics was designed by a nutritionist. The Mifflin equation [23] utilized to calculate the resting metabolic rate. Of the total calculated caloric intake, 50% was allocated to carbohydrates, 20% to protein, and 30% to fat. For participants who were overweight or obese, 500 kcal per day was deducted from the total calculated caloric intake.
To reduce the inflammatory potential of an individual’s overall diet, 45 food items [21] exhibiting pro-inflammatory and anti-inflammatory characteristics were considered for the calculation of dietary inflammatory factors (DII). To decrease dietary inflammation, foods and components with a lower DII were prioritized, while the intake of pro-inflammatory dietary components was restricted.
In general, considering the pro-inflammatory and anti-inflammatory food parameters, food sources, and the characteristics of the participants, an individualized anti-inflammatory diet was developed for both intervention groups.
Acupuncture
Acupuncture was performed for two sessions (30 min in each session) per week for a period of 8 weeks. Acupoints were selected based on the WHO standard acupuncture point locations in the Western Pacific regions, and needle manipulation techniques were based on an acupuncture textbook in China. The main acupuncture points (n = 22) were as follows: DU20, LV3 (Bilateral), SP6 (Bilateral), ST36 (Bilateral), ST25 (Bilateral), SP15 (Bilateral), PC6 (Bilateral), HT7 (Bilateral), Sishencong (EX-HN 1), Yintang (EX-HN 3), Ren4, Ren6.
Our designed protocol for “Diabetic patients with Depression” emphasized addressing various dimensions, etiologies, and symptoms of depression while avoiding factors that have been shown to increase blood sugar levels.
Sample size
In the present clinical trial with three study groups, type 2 diabetic patients with depression (mild to moderate) were examined, and our primary outcome was depression. The sample size was calculated using the G*Power analysis program. The true difference between the means was assumed to be 5 scores in depression. Considering ANOVA as a statistical test, the number of groups = 3, alpha error 0.05, power 80%, and effect size f = 0.33, 30 subjects are needed for each study group.
Outcomes
In the present study, depression was our primary outcome, and biochemical parameters (FBS, HbA1c, TC, TG, LDL-C, HDL-C, high-sensitivity C-reactive protein (hs-CRP)), anxiety, and anthropometric indices (body weight, body mass index, WC), and dietary intake were our secondary outcomes.
Recruitment
At the beginning of the study, eligible individuals were examined using the appropriate methods. Montgomery–Åsberg Depression Rating Scale (MADRS) questionnaire was administered through face-to-face interviews. Based on the scores obtained, individuals identified as experiencing mild-to-moderate depression (with scores ranging from 7 to 34) were included, while those with severe depression were referred to a psychologist. Importantly, all patients received standard treatment, and there were no changes in the dosage or type of medications throughout the study. Additionally, participants were instructed not to alter their physical activity levels during the study period. Since patients with mild-to-moderate depression typically do not receive antidepressant medications, we included only these individuals in our study.
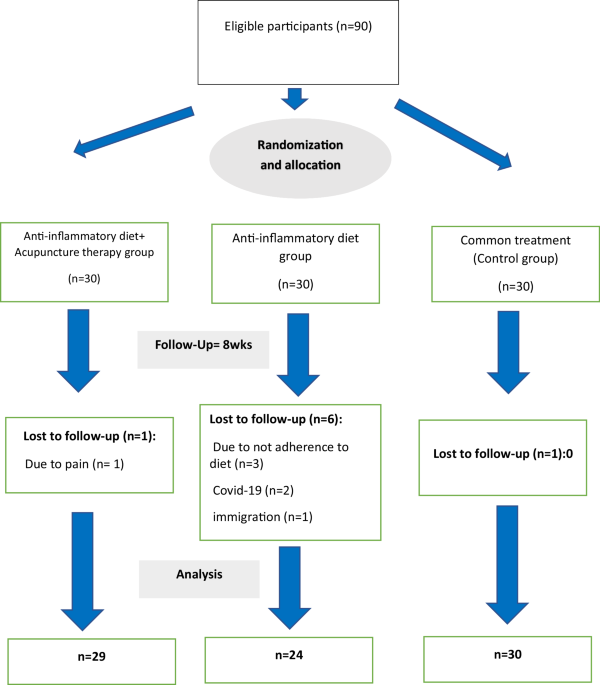
Eligible patients were selected randomly from those referred to Diabetes clinics affiliated with Tehran University of Medical Sciences. Before starting the trial, the study was explained to eligible individuals, and if they wanted to participate, they would sign the written informed consent. Randomization: before the intervention, all eligible subjects participated in a two-week run-in period. Following this period, volunteers were randomly assigned (1:1:1) to one of three groups: (i) receiving an individualized anti-inflammatory diet combined with acupuncture therapy (n = 30), (ii) receiving only an individualized anti-inflammatory diet, and (iii) receiving a standard diet with dietary recommendations (control group) (n = 30).
Notably, volunteers were randomized and allocated to each group based on the severity of depression (mild, moderate). The randomization was carried out using an online system ( Once the randomization has been made, each patient is given a code with which she/he was identified throughout the study. The randomization numbers were assigned consecutively. Randomization was not exposed to those conducting the study and was provided in sealed opaque envelopes with successive numbers. The envelope was opened after signing the informed consent form, and the patients complied with the eligibility criteria.
Blinding
Due to the nature of intervention in this study, complete blinding is not feasible. Only statistical analysis was conducted in blinded manner.
Data collection
Participants were followed for 8 weeks to examine any changes in mental health (depression, anxiety), metabolic parameters (FBS, HbA1c, TC, TG, LDL-C, HDL-C, hs-CRP), anthropometric indices (body weight, body mass index (BMI), waist circumference (WC) and body composition) and dietary intake following the intervention. At the beginning and the end of the first and second months of the study, three 24-hour dietary recalls (2 work days, 1 weekend), a physical activity questionnaire, and anthropometric measurements with standard methods were obtained. In addition, at the beginning and the end of the study, depression and biochemical parameters were assessed. Visit descriptions are provided in Table 1.
The assessments of mental health
Depression and anxiety will be examined using MADRS and the Beck questionnaire, respectively. Physical activity information was obtained through the International Physical Activity Questionnaire [24] at baseline and at the end of the study via a face-to-face interview. To examine depression, the MADRS questionnaire was used. In the present study, we applied the Persian form of MADRS; Notably, its validity and reliability were evaluated earlier [25]. The MADRS questionnaire assesses the following ten items: (1) apparent sadness, (2) reported sadness, (3) inner tension, (4) reduced sleep, (5) decreased appetite, (6) concentration difficulties, (7) lassitude, (8) inability to feel, (9) pessimistic thoughts, and (10) suicidal thoughts. Each of these ten items is rated by a clinician on a seven-point Likert scale, and the scores are summed to produce a total score ranging from 0 to 60. A higher score indicates a greater severity of depression. Usual cutoff points are as follows: 0 to 6: normal, 7 to 19: mild depression, 20 to 34: moderate depression, >34: severe depression. Accordingly, only patients with a score between 7 and 34 were included.
To assess anxiety, the Beck questionnaire (21 items) was used. Individuals with a score of 0–7 were considered to have the least anxiety, and those with a score of 26–63 were considered to have severe anxiety.
Anthropometric indices measurements
A digital weighing scale (Seca 725 GmbH & Co., Hamburg, Germany) was used for body weight measurement to the nearest 0.1 kg. Body weight and height (precision measurement: 0.5 cm) were measured using standard methods. BMI was computed by dividing the weight (kg) by the square of height 11 (m). WC was measured to the nearest 0.5 cm at the midpoint between the lower border of the rib cage and the iliac crest using a flexible tape (cm).
Biochemical measurement
At the beginning and the end of the study, after 12–14 h of fasting, 10 cc of blood was collected from the left arm arterial of study participants. Blood was taken in a sitting posture. The serum was separated by centrifugation and stored in the freezer at −70 °C until the measurement of the biochemical indicators. Notably, FBS and serum lipid levels were immediately measured after blood sampling. All biochemical variables were examined by commercial biochemical kits according to the manufacturer’s protocol.
Ethical and confidential issues
All participants provided written informed consent at the beginning of the study. All participant information is kept in a password-protected file with restricted access. To maintain participant confidence, a code identification number is used to identify data collection and forms. This research was conducted following the principles of the Declaration of Helsinki and was registered in the National Institute for Medical Research Development (NIMAD) with the Ethical Code of IR.NIMAD.REC.1398.340. The protocol was registered in the Iranian Registry of Clinical Trials (IRCTID: IRCT20110314006065N3).
Statistical methods
Descriptive information was presented by mean ± Standard deviation (SD) or number (Percent), and was compared using Mann–Whitney U and Pearson Chi-Square tests between the study groups. Shapiro–Wilk test was also applied to assess the normality assumption within the groups. Furthermore, the Generalized Estimating Equations model adjusted for age, duration of diabetes, and total energy was performed to evaluate the between-group and within-group differences in terms of fat and anthropometric indices. In addition, generalized linear models adjusted for age, weight, duration of diabetes, total energy, and the outcome baseline value were used to compare the groups in terms of depression, anxiety, biomarkers, and nutritional parameters. Both models were followed by the least significant difference post-hoc test for pairwise comparisons between or within groups. All analyses were performed through IBM SPSS version 24, and P values <0.05 were considered statistically significant.
link








